Life and the Second Law of Thermodynamics: An Unfinished Debate
The universe is, as a whole, in a high degree of disorder, which tends to increase, whereas life is in a high state of order. The elements and chemical molecules, for instance, found in nature are in a state of disorder. If they are placed in a container, they remain in that state of disorder. On the other hand, if the same elements or chemical molecules were to enter a living body, they are converted to some order; in fact, to precise orders. They take new forms of proteins, enzymes, DNA and so forth. Within a living cell they are in a superlatively high state of order. Thus, though not in the strict sense of the physicists, but, implicitly, life defies the second law of thermodynamics, writes SYED IQBAL ZAHEER.
The Second Law of Thermodynamics is known for its elusiveness. Peter Atkins (former professor of Chemistry at the University of Oxford) wrote the following in his famous 2007 work, “Four Laws that Drive the Universe”:
“The second law has a reputation for being recondite, notoriously difficult, and a litmus test of scientific accuracy.”
Considered from various perspectives, the second law can be described variously, with none yielding a meaning clearer than another. Atkins himself defined it variously. Let us start with the simplest (not from Atkins):
“The second law states that physical bodies get more disordered over time.”
A more technical definition would be:
“The Second Law of Thermodynamics says that the state of entropy of the entire universe, as an isolated system, will always increase over time. The second law also states that the changes in the entropy in the universe can never be negative.” (https://chem.libretexts.org/)
Another way is to define it as:
“The Second law of thermodynamics: The entropy of an isolated system not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium.”
And ‘entropy’ is defined as:
“…Entropy is the measure of disorder and randomness in a system.” (http://physicsforidiots.com/physics/thermodynamics/)
To make it simpler:
“The degree of randomness or disorder in a system is called its entropy.” (https://www.khanacademy.org)
In common man’s parlance: entropy is a measure of disorder; the higher the entropy, the higher the disorder, and the lower the entropy, the lower the disorder. And the universe at large is in a high state of entropy.
Entropy and the Second Law of Thermodynamics
“The degree of randomness or disorder in a system is called its entropy. Since we know that every energy transfer results in the conversion of some energy to an unusable form (such as heat), and since heat that does not do work goes to increase the randomness of the universe, we can state a biology-relevant version of the Second Law of Thermodynamics: every energy transfer that takes place will increase the entropy of the universe and reduce the amount of usable energy available to do work (or, in the most extreme case, leave the overall entropy unchanged). In other words, any process, such as a chemical reaction or set of connected reactions, will proceed in a direction that increases the overall entropy of the universe.” (https://www.khanacademy.org/science)
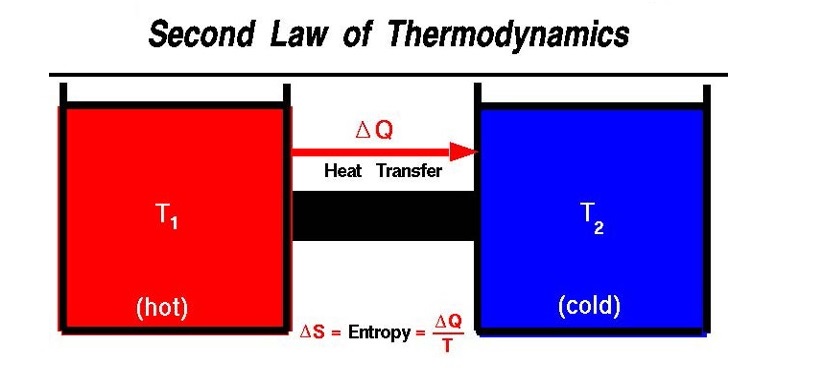 In the above, heat flows from a hot body (T1) to a cold body (T2).
In the above, heat flows from a hot body (T1) to a cold body (T2).
Heat can never flow from a cold body to a hot body.
From a yet simpler viewpoint: the universe is, as a whole, in a high degree of disorder, which tends to increase, whereas life is in a high state of order. The elements and chemical molecules, for instance, found in nature are in a state of disorder. If they are placed in a container, they remain in that state of disorder. Sometimes they will combine together, but that new combination is bound to disintegrate losing their order – if there was any.
On the other hand, if the same elements or chemical molecules were to enter a living body, they are converted to some order; in fact, to precise orders. They take new forms of proteins, enzymes, DNA and so forth. Within a living cell they are in a superlatively high state of order. The cell itself, which contains them, is, as a whole highly ordered. To be sure, when the cell drops off the body as dead, it is overcome by the law of nature, which suffers from entropy.
Thus, though not in the strict sense of the physicists, but, implicitly, life defies the second law of thermodynamics.
Scientists with atheistic leanings, however, as afraid of anything that suggests the hand of a Creator as children of roaches, generally tend to disagree with the above conclusion. They argue that, “Entropy only increases in closed systems,” whereas life is not a closed system. To give in out of some sympathy, they contend that life defying the second law of thermodynamics is – at best – only a metaphor.
To a superficial eye, there is some truth in the statement. But Atkins, whose “Four Laws that Drive the Universe” draws praise from a figure like Paul Davies, sees a far-reaching denouement of the Second Law of Thermodynamics. He writes:
 “The second law is of central importance in the whole of science, and hence in our rational understanding of the universe, because it provides a foundation for understanding why any change occurs. Thus, not only is it a basis for understanding why engines run and chemical reactions occur, but it is also a foundation for understanding those most exquisite consequences of chemical reactions, the act of literary, artistic, and musical creativity that enhance our culture.” (Four Laws that Drive the Universe, p. 49)
“The second law is of central importance in the whole of science, and hence in our rational understanding of the universe, because it provides a foundation for understanding why any change occurs. Thus, not only is it a basis for understanding why engines run and chemical reactions occur, but it is also a foundation for understanding those most exquisite consequences of chemical reactions, the act of literary, artistic, and musical creativity that enhance our culture.” (Four Laws that Drive the Universe, p. 49)
Now, if the above statement is true in the metaphoric sense, (after all, there are no equations to demonstrate how cultural enhancement, for instance, can be traced to the influence of the Second Law of Thermodynamics), then, our argument (about life defying the second law) could be similarly characterized, except that our deduction is cogently more accurate and closer to the truth.
However, is that all about it? Rather not. The following illustration should make it clearer: there is a property called life. Living bodies take in substances, otherwise disorganized, and organize them. Atoms and molecules are tuned to amino acids, proteins, DNA, and so on. These take highly organized forms; in fact, they are organized to stupendous levels that no human effort can match. What happens to the same molecules once the cell completes its cycle and drops out minus the property of life? The universe takes over and applies its law of entropy to disintegrate them. Those various highly organized molecules lose their order.
Not only are the disordered materials brought to order inside a cell, the cell itself is a highly organized governing body – organizing the machineries within it to carry out specific functions, within time – and place targets.
It is this “disorder to order” property of life which defies the Second Law of Thermodynamics.

